NCERT Solutions for Class 12 Chemistry Chapter 15 Polymers is designed and prepared by the best teachers across India. All the important topics are covered in the exercises and each answer comes with a detailed explanation to help students understand concepts better. These NCERT solutions play a crucial role in your preparation for all exams conducted by the CBSE, including the JEE.

Topics and Subtopics in NCERT Solutions for Class 12 Chemistry Chapter 15 Polymers:
| Section Name | Topic Name |
| 15 | Polymers |
| 15.1 | Classification of Polymers |
| 15.2 | Types of Polymerisation Reactions |
| 15.3 | Molecular Mass of Polymers |
| 15.4 | Biodegradable Polymers |
| 15.5 | Polymers of Commercial Importance |
NCERT Solutions For Class 12 Chemistry Chapter 15 Polymers
NCERT INTEXT QUESTIONS
15.1. What are polymers?
Ans: Polymers are high molecular mass substances (103 — 107u) consisting of a very large number of simple repeating structural units joined together through covalent bonds in a linear fashion. They are also called macromolecules. Ex: polythene, nylon 6,6, bakelite, rubber, etc.
15.2. How are polymers classified on the basis of structure?
Ans: On the basis of structure, polymers are classified into three types. These are linear chain polymers, branched chain polymers and crossedlinkedpolymers.
1. Linear chain polymers: In this case, the monomer units are linked to one another to form long linear chains. These linear chains are placed one above the other and are closely packed in space. The close packing results in high densities, tensile strength and also high melting and boiling points. High density polyethene is a very common example of this type. Nylon, polyesters and PVC are also linear chain polymers.
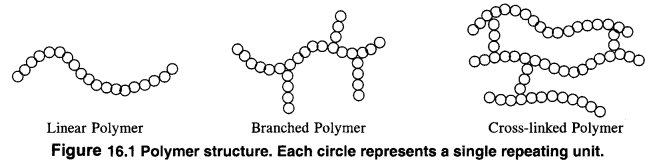
2. Branched chain polymers: In this type of polymers, the monomer units are linked to form long chains which have also side chains or branched chains of different Lengths attached to them. As a result of branching, these polymers are not closely packed in space. They have low densities, low tensile strength as well as low melting and boiling points. Some common examples of such polymers are ; low density polyethene, amylopectin, starch, glycogen etc.
3. Cross: linked polymers. In these polymers, also called net—work polymers, the monomer units are linked together to form three dimensionaL net—work as shown in the figure. These are expected to be quite hard, rigid and brittle. Examples of cross linked polymers are bakelite, glyptal. melamine formaldehyde polymer etc.
15.3. Write the names of the monomers of the following polymers:
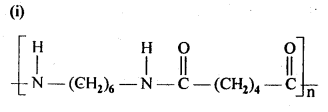
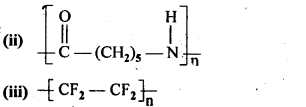
Ans: (i) Hexamethylene diamine NH2-(CH2)6NH2 and adipic acid HOOC – (CH2)4 – COOH
(ii) Caprolactum
(iii) Tetrafluoroethene F2C = CF2
15.4. Classify the following as addition and condensation polymers:
Terylene, Bakelite, Polyvinyl chloride,Polythene
Ans: Addition polymers: Polyvinyl chloride, Polythene
Condensation polymers : Terylene, bakelite.
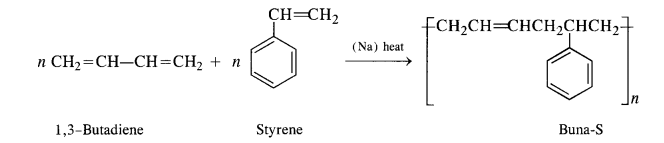
15.5. Explain the difference between Buna-N and Buna-S.
Ans: Both Buna-N and Buna-S are synthetic rubber and are co-polymers in nature. They differ in their constituents.
Buna-N: Constituents are : buta-1, 3-diene and acrylonitrile.
Buna-S: Constituents are : buta-1, 3-diene, and styrene. They condense in the presence of Na.
Buna – S: It is a co—polymer of 1. 3 – butadiene and styrene and is prepared by the polymerisation of these components in the
ratio of 3 : 1 in the presence of sodium.
Buna-N (Nitrile rubber): h is a co-polymer of buta-1. 3-diene and acrylonitrile. It is formed as follows:
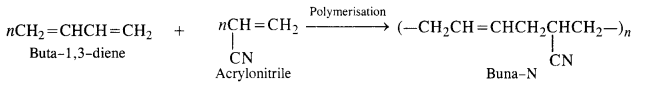
15.6. Arrange the following polymers in increasing order of their intermolecuiar forces.
(i) Nylon 6,6, Buna-S, Polythene
(ii) Nylon 6, Neoprene, Polyvinyl chloride
Ans: On the basis of intermolecuiar forces, polymers
are classified as elastomers, fibres and plastics. The increasing order of intermolecuiar forces is: Elastomer < Plastic < fibre.
Thus, we have
(i)Buns-S < Polythene < Nylon 6,6
(ii)Neoprene < Polyvinyl chloride < Nylon 6.
NCERT EXERCISES
15.1. Explain the terms polymer and monomer.
Ans: Polymers are high molecular mass substances consisting of a very large number of simple repeating structural units joined together through covalent bonds in a regular fashion. Polymers are also called macromolecules. Some examples are polythene, nylon-66, bakelite, rubber, etc. Monomers are the. simple and reactive molecules from which the polymers are prepared either by addition or condensation polymerisation. Some examples are ethene, vinyl chloride, acrylonitrile, phenol and formaldehyde etc.
15.2. What are natural and synthetic polymers ? Give two examples of each.
Ans:
1. Natural polymers: The polymers which occur in nature mostly in plants and animals are called natural polymers. A few common examples are starch, cellulose, proteins, rubber nucleic acids, etc. Among them, starch and cellulose are the polymers of glucose molecules. Proteins are formed from amino acids which may be linked in different ways. These have been discussed in detail in unit 15 on biomolecules. Natural rubber is yet another useful polymer which is obtained from the latex of the rubber tree. The monomer units are of the unsaturated hydrocarbon 2-methyl-i, 3-butadiene, also called isoprene.
Example of natural polymers: Natural rubber, cellulose, nucleic acids, proteins etc.
2. Synthetic polymers: The polymers which are prepared in the laboraroiy are called synthetic polymers. These are also called man made polymers and have been developed in the present century to meet the ever increasing demand of the modem civilisation.
Example of synthetic polymers: Dacron (or terylene), Bakelite, PVC, Nylon-66, Nylon-6 etc.
15.3. Distinguish between the terms homopolymer and copolymer and give an example of each.
Ans: Polymers whose repeating structural units are derived from only one type of monomer units are called homopolymers, e.g., PVC polyethene, PAN, teflon, polystyrene, nylon- 6 etc.
Polymers whose repeating structural units are derived from two or more types of monomer molecules are copolymers, e.g., Buna-S, Buna-N, nylon-66, polyester, bakelite.
15.4. How do you explain the functionality of a monomer?
Ans: Functionality of a monomer implies the number of bonding sites present in it. For example, monomers like propene, styrene, acrylonitrile have functionality of one which means that have one bonding site.
Monomers such as ethylene glycol, hexamethylenediamine, adipic acid have functionality of two which means that they have two bonding sites.
15.5. Define the term polymerisation?
Ans: It is a process of formation of a high molecular Sol. mass polymer from one or more monomers by linking together a large number of repeating structural units through covalent bonds.
15.6. Is (-NH — CHR—CO-)n a homopolymer or copolymer?
Ans: It is a homopolymer because the repeating structural unit has only one type of monomer, i.e., NH2—CHR—COOH.
15.7. In which classes, are the polymers classified on the basis of molecular forces?
Ans: Polymers are classified into four classes on the basis of molecular forces. These are:
elastomers, fibres, thermoplastic polymers and thermosetting polymers.
1. Elastomers: In these polymers, the intermolecular forces are the weakest. As a result, they can be readily stretched by applying small stress and regain their original shape when the stress is removed. The elasticity can be further increased by introducing some cross – links in the polymer chains. Natural rubber is the most popular example of elastomers. A few more examples are of: buna-S, buna-N and neoprene.
2. Fibres: Fibres represent a class of polymers which are thread-like and can be woven into fabrics in a number of ways. These are widely used for making clothes, nets, ropes, gauzes etc. Fibres possess high tensile strength because the chains possess strong intermolecular forces such as hydrogen bonding. These forces are also responsible for close packing of the chains. As a result, the fibres are crystalline in nature and have aJso sharp melting points. A few common polymers belonging to this class are nylon – 66, terylene and polyacrylonitrile etc.
3. Thermoplastics: These are linear polymers and have weak van der Waals forces acting in the various chains and are intermediate of the forces present in the elastomers and in the fibres. When heated, they melt and form a fluid which sets into a hard mass on cooling, Thus, they can be cast into different shapes by using suitable moulds. A few common examples are polyethene and polystyrene polyvinyls etc. These can be used for making toys, buckets, telephone apparatus, television cabinets etc.
4. Thermosetting plastics: These are normally semifluid substances with low molecular masses. When heated, they become hard and infusible due to the cross-linking between the polymer chains. As a result, they also become three dimensional in nature. They do not melt when heated. A few common thermosetting polymers are bakelite, melamine-formaldehyde, urea-formaldehyde and polyurethane etc.
15.8. How can you differentiate between addition and condensation polymerisatiop?
Ans: In addition polymerization, the molecules of the same or different monomers simply add on to one another leading to the formation of a macromolecules without elimination of small molecules like H2O, NH3 etc. Addition polymerization generally occurs among molecules containing double and triple bonds. For example, formation of polythene from ethene and neoprene from chloroprene, etc. In condensation polymerisation, two or more bifunctional trifimctional molecules undergo a series of independent condensation reactions usually with the elimination of simple molecules like water, alcohol, ammonia, carbon dioxide and hydrogen chloride to form a macromolecule. For example, nylon-6,6 is a condensation polymer of hexamethylenediamine and adipic acid formed by elimination of water molecules.
15.9. Explain the term copolymerisation and give two examples.
Ans: When two or more different monomers are allowed to polymerise together the product formed is called a copolymer, and the process in called copolymerisation. Example, Buna-S and Buna-N. Buna- S is a copolymer of 1, 3- butadiene and styrene while Buna-N is a copolymer of 1,3-butadiene and acrylonitrile.
15.10. Write the free radical mechanism for the polymerisation of ethene.
Ans:
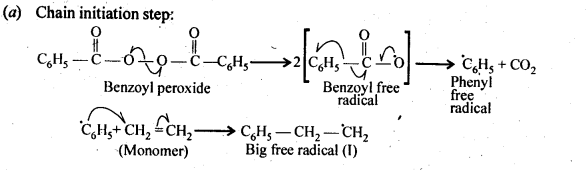
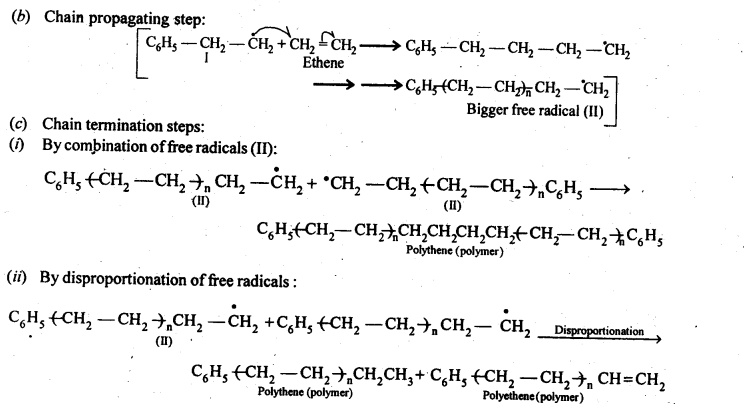
15.11. Define thermoplastics and thermo setting polymers with two examples of each
Ans: Thermoplastics polymers are linear polymer which can be repeatedly melted and moulded again and again on heating without any change in chemical composition and mechanical strength. Examples are polythene and polypropylene.
Thermosetting polymers, on the other hand, are permanently setting polymers. Once on heating in a mould, they get hardened and set, and then cannot be softened again. This hardening on heating is due to cross- linking between different polymeric chains to give a three dimensional network solid. Examples are bakelite, melamine-foimaldehyde polymer etc.
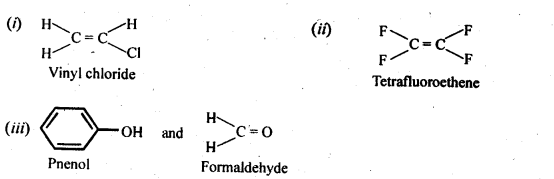
15.12. Write the monomers used for gettingThe following polymers:
(i) Polyvinylchloride
(ii) Teflon (iii) Bakelite
Ans:
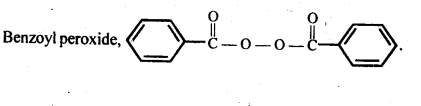
15.13. Write the name and structure of one of the common initiators used in free radical addition polymerisation.
Ans:
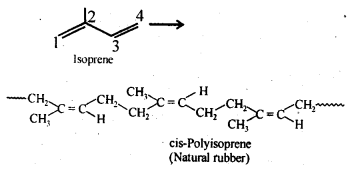
15.14. How does the presence of double bonds in rubber molecules influence their structure and reactivity?
Ans: Natural rubber is cis-polyisoprene and is obtained by 1, 4-polymerization of isoprene units. In this polymer, double bonds are located between C2 and C3 of each isoprene unit. These cis-double bonds do not allow the polymer chains to come closer for effective interactions and hence intermolecular forces are quite weak. As a result, natural rubber, i.e., cis-polyisoprene has a randomly coiled structure not the linear one and hence show elasticity.
15.15. Discuss the main purpose of vulcanisation of rubber.
Ans: Natural rubber has the following disadvantages:
(a) It is soft and sticky and becomes even more so at high temperatures and brittle at low temperatures. Therefore, rubber is generally used in a narrow temperature range (283-335 K) where its elasticity is maintained.
(b)It has large water absorption capacity, has low tensile strength and low resistance to abrasion.
(c)It is not resistant to the action of organic solvents.
(d)It is easily attacked by oxygen and other oxidising agents. .
To improve all these properties, natural rubber is vulcanised by heating it with about 5% sulphur at 373-415 K. The vulcanized rubber thus obtained has excellent elasticity over a larger range of temperature, has low water absorption tendency and is resistant to the action of organic solvents and oxidising agents.
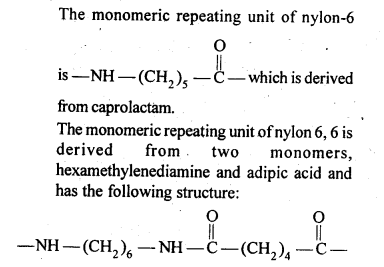
15.16. What are the monomeric repeating units of Nylon-6 and Nylon 6,6?
Ans:
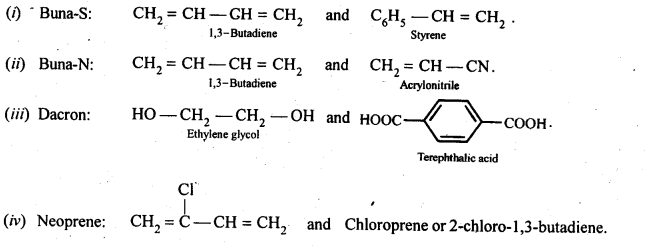
15.17. Write the names and structures of the monomers of the following polymers:
(i) Buna-S (ii) Buna-N (iii) Dacron (iv) Neoprene
Ans:
15.18. Identify the monomer in the following polymeric structures:
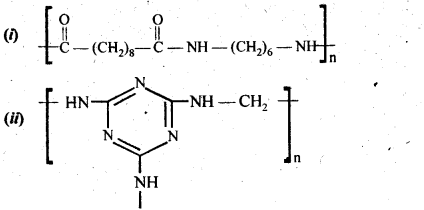
Ans:
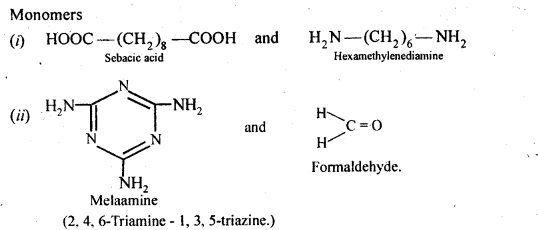
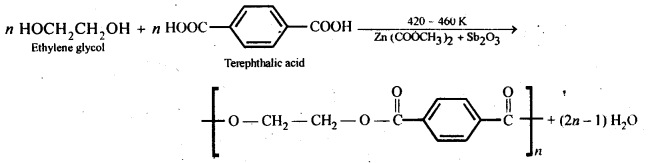
15.19. How is dacron obtained from ethylene glycol and terephthalic acid?
Ans: Dacron is obtained by condensation polymerization of ethylene glycol and terephthalic acid with the elimination of water molecules. The reaction is carried out at 420 – 460 K in presence of a catalyst consisting of a mixture of zinc acetate and antimony trioxide.
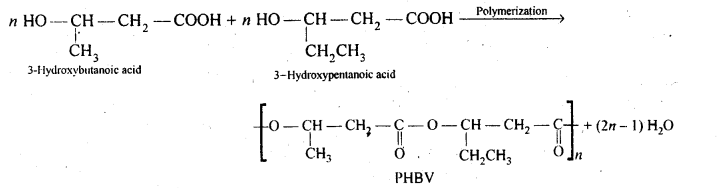
15.20. What is a biodegradable polymer ? Give an example of a biodegradable aliphatic polyester.
Ans: Polymers which disintegrate by themselves over a period of time due to environment degradation by bacteria, etc., are called biodegradable polymers. Example is PHBV, i. e., Poly-β-Hydroxybutyrate-co-β- Hydroxyvalerate.
Download the PDF of NCERT Solutions from Here
Class 12 Chemistry NCERT Solutions
- Chapter 1 The Solid State
- Chapter 2 Solutions
- Chapter 3 Electro chemistry
- Chapter 4 Chemical Kinetics
- Chapter 5 Surface Chemistry
- Chapter 6 General Principles and Processes of Isolation of Elements
- Chapter 7 The p Block Elements
- Chapter 8 The d and f Block Elements
- Chapter 9 Coordination Compounds
- Chapter 10 Haloalkanes and Haloarenes
- Chapter 11 Alcohols Phenols and Ethers
- Chapter 12 Aldehydes Ketones and Carboxylic Acids
- Chapter 13 Amines
- Chapter 14 Biomolecules
- Chapter 15 Polymers
- Chapter 16 Chemistry in Everyday Life
More Resources for NCERT Solutions Class 12: